-
Sterically Encumbered Diphosphaalkenes and a Bis(diphosphene) as Potential Multiredox-Active Molecular Switches: EPR and DFT Investigations
C. Dutan, S. Shah, R.C. Smith, S. Choua, T. Berclaz, M. Geoffroy and J.D. Protasiewicz
Inorganic Chemistry, 42 (20) (2003), p6241-6251


DOI:10.1021/ic030079j | unige:3507 | Abstract | Article HTML | Article PDF
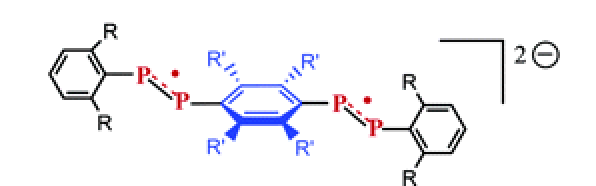
The reduction products of two diphosphaalkenes (1 and 2) and a bis(diphosphene) (3) containing sterically encumbered ligands and corresponding to the general formulas ArâX==YâArââY==XâAr, have been investigated by EPR spectroscopy. Due to steric constraints in these molecules, at least one of the dihedral angles between the CXYC plane and either the Ar plane or the Arâ plane is largely nonzero and, hence, discourages conformations that are optimal for maximal conjugation of P==X (or P==Y) and aromatic Ï systems. Comparison of the experimental hyperfine couplings with those calculated by DFT on model systems containing no cumbersome substituents bound to the aromatic rings shows that addition of an electron to the nonplanar neutral systems causes the X==YâArââY==X moiety to become planar. In contrast to 1 and 2, 3 can be reduced to relatively stable dianion. Surprisingly the two-electron reduction product of 3 is paramagnetic. Interpretation of its EPR spectra, in the light of DFT calculations on model dianions, shows that in [3]2- the plane of the Arâ ring is perpendicular to the CXYC planes. Due to interplay between steric and electronic preferences, the ArâX==YâArââY==XâAr array for 3 is therefore dependent upon its redox state and acts as a âmolecular switchâ.